Max Planck and his Packets

Max Planck
The Grand Old Man
During a recent bronze-casting course at a well known art school, the students were heating up the ceramic molds to the requisite 1600 degrees Fahrenheit. Everything has to be that hot or the bronze will freeze before it gets into all the nooks and crannies of the "shells".
When the oven was shut off and the sled with the molds pulled out, the inside of the shells were glowing a dull orange. The outside of the molds were their usual grayish white. One of the students said, "Huh! Blackbody radiation."
Blackbody radiation is - to use the usual egghead definition - "the electromagnetic radiation given off by a theoretical blackbody, its average frequency increasing as the blackbody's temperature increases". Naturally that sends you to the definition of a "blackbody". Then you learn a blackbody is "an ideal body or surface that completely absorbs all radiant energy falling upon it with no reflection and that radiates at all frequencies with a spectral energy distribution dependent on its absolute temperature".
All that gobbledy-gook means is that if you heat something up it begins to glow. If the blackbody is hot enough, the emitted radiation is visible light. And that's what the ceramic shells were doing.
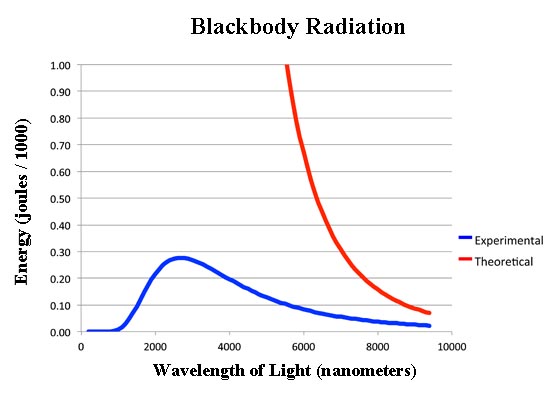
What had interested physicists in the 19th century was not just that hot things glowed, but the spectrum of how they glowed. That is, there was a certain intensity of light given off at a given wavelength. The theory was a simple formula called the Rayleigh-Jeans equation which is:
I = (k × T) / λ
... where I is equal to the energy of the radiation at the wavelength λ. The symbol k is a constant which we will not bother deriving.
The problem - as you can see, if you click on the image - was the theory didn't work too well. In fact it stunk.
Not only does the theory stink but it's so far off as to be laughable. According to the theory, the radiation coming out of the opening should not be a nice dull orange. As you can see at lower wavelengths, the intensity - that is the energy - should get larger and larger without any limit. That means once the art students opened the oven and pulled out the molds, they should have been zapped with ultra high energy gamma rays!
Well, obviously that wasn't happening since the students remained hale and healthy and managed to cast their bronze statues.
The discrepancy between theory and experiment bothered the 19th century scientists. And that's where Max Planck came in.
Anyone who has taken college or even high school level physics or chemistry has heard of (and probably forgotten) Max Planck. In 1900 he was a well known physicist who was teaching at the University of Berlin, then patriotically called the Friedrich Wilhelms Universität.
Max was born on April 23, 1858 in Kiel. His dad was a law professor at Munich but Max's interests turned toward math and science. So he studied at the Universities of Munich and Berlin and in 1879 got his doctorate at Munich where he began to teach.
This was the time where modern thermodynamics and statistical mechanics were hot topics. The two subjects were (and are) closely related but while the basic thermodynamic equations are often simple, the math for statistical mechanics can be horrendous. As this was the day before computers you had to be well versed in calculus and differential equations to even get to the batter's box, much less first base.
By 1900, Max had published a number of papers but none were what you would call earth-shattering. Max was 42 and long past the age where most famous physicists make their most important discoveries..
But one thing kept bugging him. The problem of the hot ceramic molds. That is the blackbody radiation.
Actually, you don't need to shine radiation on a blackbody to make it emit blackbody radiation. Anything that absorbs energy and then begins to radiate is emitting blackbody radiation. Blackbody radiation is everywhere. For instance, turn on an old fashioned incandescent lightbulb and the wire absorbs the electrical energy and begins to glow. You'll get a blackbody spectrum.
But the problem still remained. Theory would not predict the intensity of light that came out of a black body. You needed an equation that started out at zero, went up to a higher value, and then dropped off and headed back down to zero.
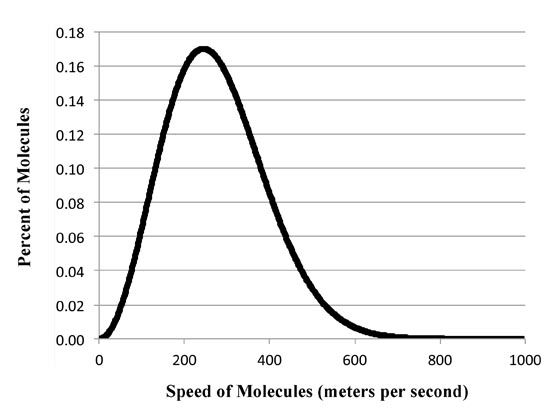
Max struggled with the problem, trying various theoretical ways to get the equation. Then a professor named Ludwig Boltzmann - who actually knew Max pretty well - had been able to show that such this type of curve arises when you plot the energy of gas molecules bouncing around a room as a function of temperature.
Ludwig ideas was you grouped the molecules by their energy. Then by pure probability the likelihood for all molecules to be moving very slowly isn't likely. Nor that they are all moving very fast very likely. So at the extremes of the graph the values will be very close to zero and in the middle of the graph at some place you'll get a maximum. So you get the right curve:
Now, the reason Ludwig's theory worked was because he was dealing with only a finite number of individual molecules. So the energy is distributed as packets of energy corresponding to the energy of each molecules. Average them up and you get what we now call the Boltzmann distribution.
So Max did some thinking. There was a trick - and he saw it as a trick - that if you limited the energy of light to having only discrete values then light would act more like the molecules. So he hypothesized that light energy wasn't continuous but discrete:
E = nhν
... where n = 1, 2, 3, ... on up.
E of course is the energy of the light, h is an empirically derived constant (commonsensically called Planck's constant), and ν is the Greek letter, pronounced nu.
And what, you ask, is ν?
Oh, not much. What's ν with you?
NyeahahahahahahaHAHAHAHAH!!!!!!!!!
.Seriously, ν is the frequency of the light. The value of h is quite small with 6.626,069,83 × 10-34 joules-sec. It tells you how much energy is in the light packet.
The light "packet" or particle is, of course, the famous photon. Today everyone thinks of light coming in packets as no big deal and quantum theory is accepted by anyone with even the smallest smattering of education.
Now it's not that difficult to derive Ludwig's equation although a bit lengthy (you can see how it's done if you click here). Max's derivation is also straightforward but a little more complex. Suffice it ot say using Ludwig's distribution and by hypothesizing light was absorbed and emitted in individual quanta, Max was able to derive the experimental spectrum for blackbody radiation that fit the theory. If you want to get into a bit of the nitty-gritty, you can see Max's derivation in a new window if you click here.
At first Max didn't believe the energy packets - the photons - were real. He thought he had just discovered some slick mathematical trick that forced the light to follow the proper equation. In fact, he hoped eventually someone would reconcile the trick with reality and show that light really wasn't in little bunches.
However, in 1905, a chap named Albert Einstein showed that having light move around as discrete photons explained what was called the photoelectric effect. That is, if you shone light on certain metals they gave off electrons. Albert proved that you had to believe in photons if you believed in the photoelectric effect. He wrote this all up in a paper that landed him a Nobel Prize in 1921.
Albert had started the ball rolling. In 1912, Neils Bohr showed how Max's quantized energy could be applied to atoms in a way that would explain atomic spectra - that is the way atoms absorb and emit light. Neils's work landed him a Nobel.
Then Louis De Broglie in France showed that Max's equation told us why electrons acted like waves if they were sent through crystals (Louis also won a Nobel). Louis also got a Nobel Prize.
And in 1926, Werner Heisenberg and Erwin Schrödinger, independently developed quantum mechanics using Max's equation as a starting place. Werner and Erwin's (they both got Nobel prizes, too). So you can justifiably argue that Max's equation - simple as it is - is the most important scientific formula in history. And everyone was getting Nobel prizes for Max's work!
But don't despair. Max himself got the Nobel Prize in 1918.
After we read that Max came up with quantum theory, most people read nothing more about him. It's almost as if he dropped from the scientific scene. That's far from the case.
One of Max's claims to fame is he was the associate editor of the journal, Annalen der Physik from 1895 until it temporarily went out of business during World War II. As a result he was involved in the publication of some of the most important papers including the 1905 papers of Albert Einstein on Brownian motion (which established the reality of atoms and molecules), the photoelectric effect, and his special theory of relativity. Later in 1919, Albert published his general theory of relativity in the same journal.
Professionally things went pretty smoothly for Max - until the year 1933. That was when Maria Schicklgruber's grandson wound up as Chancellor of Germany. Immediately he began implementing his promises to eliminate - quote - "Non-Aryan" - elements from German society. To this end the Reichstag passed what are called the Nuremberg Laws which removed all people of Jewish ancestry from professional jobs.
Although Max had retired from his professorship in 1928, he maintained his leadership role as editor of Annalen and also in the Kaiser Wilhelm Society and the Prussian Academy of Sciences. Suddenly Max found himself in a tight spot. He not only had a number of Jewish friends and colleagues - he had been responsible for getting Albert Einstein to move to the University of Berlin and had helped Lise Meitner be appointed as a research associate which later led to her professorship at Berlin. But now in his seventies, Max and other German scientists were forbidden to travel abroad and were told to replace anyone with even a single Jewish grandparent with people with acceptable Bestätigungen. Max, though, did what he could, even meeting personally with Hitler to try to argue against removing Jewish scientists from their university posts - without, of course, any success.
We mentioned Max's professional life ran fairly smoothy all things considered. That wasn't true for his personal life. Both his wife and two daughters had died by 1919, and his oldest son had been killed in World War I. Then in 1944 his youngest son, Erwin, having been implicated in the 1944 bomb plot against Hitler, was executed by the Gestapo.
During the Second World War Max's house in Berlin had been destroyed by Allied bombs. In 1945 Max - now in his late-80's - went to live with a relative in Göttingen. He died two years later.
References
The Dilemmas of An Upright Man: Max Planck as Spokesman for German Science, John Heilbron, University of California Press, 1986.
The American Heritage Dictionary of the English Language, American Heritage, Fifth Edition.
"NIST-4 Watt Balance Weighs in on Planck's Constant", Physics World, June 29, 2016.
"The Particle That Broke a Cosmic Speed Limit", Natalie Wolchover, Quanta Magazine, May 14, 2015
"Quanta, Matter, and Change: A Molecular Approach to Physical Chemistry", Peter Atkins, Julio de Paula, Ronald Friedman, Oxford University Press, 2009.
"The Odd Couple: Boltzmann, Planck and the Application of Statistics to Physics (1900 - 1913)", Massimiliano Badino, Annalen der Physik, Issue 2-3, pp. 81-101, 2009.
"Max Planck", John O'Connor and Edmund Robertson, MacTutor History of Mathematics, St. Andrew University, Web.
"Ludwig Boltzmann", John O'Connor and Edmund Robertson, MacTutor History of Mathematics, St. Andrew University, Web.