Lise Meitner, Otto Hahn, and the Rest of the Gang

Lise Meitner
She did the figgerin'.
Lise Meitner was born in Vienna in 1878. As a young girl she showed exceptional ability in math and science, and her parents encouraged her to get as good an education as possible.
The trouble was that for millennia there had been limited opportunities for women who wanted to go for something other than the Kinder, Küche, Kirche Unsinn that was de rigueur in Deutschland (and all other countries for that matter). Once the girls hit their teens the standard practice was to discontinue their education (if any), get married, and have kids. Among the few jobs available for women was teaching, and for this you had to go to an all-girls private höhere Töchterschule.
But in the late 19th century things began to change. With the Napoleonic Wars, the American Civil War, and the Crimean War - that is, wars in the modern sense - the men were away for years at a time. So women were forced to take on normally male-dominated jobs, not just on the farm but also in the cities. And some women like Florence Nightingale and Clara Barton became heroes for doing jobs that made strong men quail.
Still education was still largely gender-segregated. Most colleges - Oxford, Cambridge, and the like - were all male. So occupations requiring special degrees were largely closed to women.
But there was a glitch in the system. In some places the tradition of the girls not attending college was so strong that the universities had never bothered to specifically exclude women. This situation was similar to that on the professional golf circuit. At first there was no specific prohibition against women playing in the tournaments. But when Babe Zaharias began qualifying for the men's tournaments the fellows put an end to that nonsense in a hurry.

Babe Zaharias
In a Similar Situation
Instead what kept the girls out was the curriculum. Girls' schools simply avoided the subjects that were prerequisites for university admission. Without the right classes the girls wouldn't be able to pass the entrance exams.
Then some of the girls had a bright idea. They would get their parents to hire private tutors. Then they could learn traditionally masculine subjects like math, science, history, and philosophy. Then when the college exams were scheduled at the boys' gymnasium - a particularly tough college preparatory high school - the girls would just walk in and take the tests.
And how did the colleges handle the onslaught of the ladies?
Well, like Perchik told the rabbi, if it isn't specifically forbidden, it must be all right. And in Lise's day - the turn of the 19th to the 20th century - the schools were largely administered by the professors themselves. With the student numbers being so low - a college might have a total of 200 students - you simply didn't need the multitude of deans, associate deans, assistant deans, directors, assistant directors, and vice-presidents that are de rigueur now. Once the girls passed the exams, all they needed was to get the profs to give them the OK to attend the class.
This was how Lise matriculated at the University of Vienna in 1901. Although she still liked math, she picked physics as her major. At that time virtually all the physics classes were taught by the famous Ludwig Boltzmann who even today is considered one of the titanic figures of modern physics. But Ludwig was also a rare bird and he couldn't have cared less whether the student sported trousers or a dress. Indeed, he seemed happy to have both in his lectures.
The picture many have of the German universities is a place where the formidable and monocled Herr Professor presides over his trembling and terrified students with his blustering delivery on incomprehensible subjects. But this was Vienna, the most relaxed of Germanic cities (in fact, it was in Austria), and Ludwig was more relaxed than most of the professors. He was also known for his enthusiasm for the subject, an enthusiasm that alternated between bouts of severe depression mixed with occasional suicide attempts. Today he'd be considered to be bipolar, or in older language, manic-depressive. But evidently he was a good teacher.
Lise followed the physics curriculum, and all in all things weren't too bad. Sure, she found some of her fellow students - who were men - were rather snooty, but others were friendly and congenial enough. Some even went out of their way to make Lise - who was normally a very shy girl - feel comfortable. The only negative effect of her following a traditionally male dominated curriculum is somewhere along the line she picked up a traditionally male dominated habit - smoking.
Lise did well and in 1905, she graduated near the top of her class. The question was what to do now. She certainly had no real interest in teaching at some girls' school. Besides physics was too interesting to cut off her studies. And so she enrolled in Vienna's Ph. D. program.
Modern college students often hear their professors lament how easy kids have it now. You can get a professor who berates his class ad nauseam on how lazy they are. Why, when he was their age, by Juste, he had read all volumes of Annals of Physics and Applied Physics Letters. Huh! Today's kids barely read a fraction of the published research.
Of course, when he graduated there were 50 issues of Annals of Physics and six issues of Applied Physics and he could get by by perusing two or three journals a year. Today there are 700 issues of Annals and over 600 of Applied and the number of other journals has ballooned up to such an exponential degree that a lot of people don't even know they exist.
Above all, in the 19th and early 20th century the colleges were not what we have today, and sometimes it's hard to figure out how the students spent their time. When J. R. R. Tolkien entered Oxford he found he had already read most of the requirements for his degree. C. S. Lewis complained that the literature majors could complete their assignments while lolling in their baths. And Albert Schweitzer seems to have studied what he wanted with only a general direction from the teachers. Go back a bit further and things were even looser. At the University of Virginia in the 1830's, the students had so much freedom they spent most of their time drinking, gambling, and having the occasional riot.
If getting an education was less formidable than today, it was also less expensive. Many professors - even at places like Oxford, Cambridge, or Harvard - had earned degrees no higher than a Bachelor's - essentially cutting costs in half. There was even one poor Yorkshire lad who was able to enter a major English university by taking free night courses. After getting his degree, he then went on to Germany, got a Ph. D., and landed a job at Oxford. And all before the age of 30.
Sure, some people are smart. But that smart?
Well, yes and no. Certainly that fellow was smart. But before World War I, getting a Ph. D. did not necessarily require the extended commitment it does today. Nowadays gathering data for a thesis can take years. But in the early 20th century, it was common to complete the work in a few months.
Ha? (To quote Shakespeare.) What was that you said?
It was common to complete doctoral research in a few months. And getting a Ph. D. in a year was not unusual.
That's why Lise didn't do graduate work with Ludwig. He had gone off for a few months to lecture in America. So she decided to work under the direction of Franz Exner, another of Vienna's well-established and congenial physics professors. Her thesis was a test of Maxwell's equations, and in 1906, Lise graduated summa cum laude, the second woman at the University of Vienna to get a doctorate.
The question, remained. What to do now? True, it was not unprecedented that women could hold major university professorships. Marie Curie had won a Nobel Prize three years earlier for her doctoral thesis - work that required at least comparable effort of the modern Ph. D. - and had been appointed professor at the University of Paris to succeed her husband Pierre who had been killed in a traffic accident. But all in all, job opportunities for lady physicists were few and far between.
Then, as now, one way to mark time was to go for post-grad studies. One of Ludwig's assistants, Stefan Meyer, suggested he and Lise study the new field of radioactivity. So they began to collaborate and Lise figured she could continue to work with Ludwig when he got back from America.

Marie Curie
A Modern Ph. D.
But that year the last of Ludwig's suicide attempts was successful. So Lise kept working with Stefan.
Although radioactivity was still rather mysterious, people had begun to get a general idea of what was going on. By the time Lise graduated everyone pretty much knew that radioactivity was the ejection of particles or the emanation of electromagnetic radiation from the atoms.
What they didn't know was the hazards of the field. During her research Lise noticed that the surfaces of the lab had also become radioactive - even if they were some distance from the equipment. The surprise is not that Marie Curie and her daughter, Irene, lived relatively short lives, but that others, like Lise, lived so long.
By the end of 1907 - a year after graduating - Lise had not only published her thesis research but had gone on to complete a total of three further independent projects and published her findings. Even today that's a respectable showing for an individual researcher.
If today's graduate students look with longing at a program where it was common to get a doctorate in a year, their professors give a sigh of envy when reading about the process of publication. You'd send in the paper and the editor would decide if he'd publish it. None of that two or more referees nonsense and six months or more waiting to hear from them. If the editor couldn't decide by himself whether the paper merited publication, he had no business being an editor. Even the prestigious journal Nature had no formal refereeing system until 1967.
Helping the facility of publication - and this is really strange to modern researchers - is how the editors sometimes had to scramble to find papers to fill out their journal. If you submitted a paper - unless it had glaring errors or the editor thought it unsound - it was pretty much guaranteed to appear.
We do not suggest that Lise's research lacked merit and when she graduated she was certainly qualified for a university post. She could, had she wished, take the obvious path and teach. But Lise wanted to do research.
And the best place for research was Berlin where Max Planck was teaching at the Friedrich-Wilhelms University. That's what they called the University of Berlin back then.
In 1907, Max was one of the most famous physicist in the world. He had caused a stir in 1900 for proposing the quantum theory of light. That is, electromagnetic radiation was emitted and absorbed as discrete packets. Max's findings served as the basis for a revolution in science which was not that far off and anyone who has taken a high school chemistry or physics course has heard of Max.
Once in Berlin, Lise went to Max's office and asked if she could attend his lectures. Now Max had a reasonably traditional attitude regarding women. Sure, if they showed unusual ability, let them into the University, but most of the ladies should stay home with the kids. Still, all in all, Lise found Max, although a bit distant, friendly enough. He even invited her to visit him and his wife at their home.

Max Planck
He was fairly traditional.
Lise, though, did have her doctorate, and so Max had no objection to her attending his lectures. But she also wanted to carry out experimental work and decided to see if any of the professors would let her use their labs.
She first spoke to Professor Heinrich Rubens whose lab space was at a premium. But he introduced her to Otto Hahn, a new faculty member who was the same age as Lise. Otto said he would be happy to share his space with Lise, and Otto and Lise continued to work together for the next thirty years.
At this point we need the pause, if not that refreshes, then that educates. Although Lise worked with Otto throughout most of her career, she also conducted her own independent research. But after she and Otto became internationally known, the German press almost always referred to her as Otto's Mitarbeiterin.
Now those with a smattering of German would likely translate this word - which literally means "with-worker" - as "business partner", "collaborator", or "colleague". These English terms are in fact accurate enough in describing Lise and Otto's relationship. But that's not what Mitarbeiterin means.
In German calling Lise eine Mitarbeiterin actually means she was an "assistant" or "employee" of Otto. Such use of the word, of course, is incorrect, and this designation did irritate Lise considerably. In her later life she wrote Otto some rather (for her) petulant letters about the matter as if she thought he hadn't done enough to clear up the confusion. Personally, though, and even during more trying times, she always remained good friends with Otto and his family.

Otto Hahn
He was happy to collaborate.
In 1908 Lise and Otto published their first papers. One reported that in some radioactive atoms, the radioactivity shot out with so much force that the new atoms got knocked to the surface of the sample. This phenomenon was called "recoil", and because the new atoms accumulated at the surface, it was possible to isolate the new element in high purity. These papers attracted considerable attention and Lise's name became known to an international audience. When she met Ernest Rutherford, who would soon deduce the correct structure of the atom, he did a double take. "Oh!" he said. "I thought you were a man."
So within two years of getting her doctorate, Lise was a famous scientist. But there was a wee, tiny little glitch. Lise wasn't getting paid. True, such "volunteers" were common in late 19th and early 20th century labs, and there were even some professors who didn't get a salary either. For years, J. Willard Gibbs, the famous American physicist, lived off his family's wealth, and Yale finally offered him a salary only when another university tried to lure him away.
Instead Lise had to get by on a modest allowance sent by her mom and dad. Naturally she had to live rather frugally but she did manage to put enough aside for her cigarettes.
As in Vienna, a lot of the guys in Berlin were nice, but others acted like jerks when they learned that a woman was in their midst. One editor of an encyclopedia had read some articles by an "L. Meitner". He was impressed and wrote her asking for some contributions. But when he found "Doktor Meitner" was eine Dame and not ein Herr, he snorted he wouldn't publish anything by a woman.
In 1912, Emil Fischer, the famous and (even now) important chemist helped wrangle Otto a job as a research associate at the new Kaiser Wilhelm Institute of Chemistry. The job carried the courtesy title of "Professor" plus a salary of 5000 marks. At the-then exchange rate, that was $1250 per year which is now about $75,000 if you do the calculations.
Lise could come along, of course. But as long, the administrators said, as she didn't ask for a salary.
This stipulation caused a major problem since Lise's dad had died a year before. So it looked like she'd have to return to Vienna. Fortunately Max Planck stepped in and gave her what was essentially a teaching assistantship.
But Lise did not get a Ph. D. just to be a TA, and Max and the others had recognized that Lise was someone they didn't want to loose. So within a year Emil had appointed her as a research associate, which technically was the same job as Otto's. True, the pay wasn't great, but at least Lise was now solvent.
The years when Lise was most active - 1905 to 1945 - were among the most important decades in history. This was the time - although representing only about 0.001% of the span of human existence - where we went from not really knowing what was going on to pretty much knowing what's going on.
Even by the start of the 20th century, no one was sure what atoms were. A lot of people weren't even convinced they existed. Maybe they were simply a mathematical convenience - like the epicycles Tycho and Copernicus used for their solar systems. So if people weren't even sure atoms existed, you can also bet that no one had any idea of how they were put together.
But by 1945, anyone of education knew that atoms existed. And everyone knew that far from being indivisible, atoms could be split, smashed, and transmuted into something else.
But the first half of the 20th century was not just when people figured out the way the world worked. This was where we saw the transformation of Science into Big Science.
Some people say that it was Ernest Rutherford and his experiments on atomic structure that required increasingly complex equipment that ushered in the era of Big Science. Without saying this is entirely incorrect, probably the real instigator of the Bigness of Big Science was Big Ernest Lawrence when he invented the cyclotron.
But Big Science required Big Money, and before the 1950's, government grants were few and far between. Fortunately, Ernest, both a good salesman as well as a good scientist, was able to go to private philanthropists for most of his cash.
One of the most unusual examples of private scientific funding was in Denmark. In Copenhagen the family that ran the Carlsberg Brewery had been giving money to scientists since the 19th century. The Institute of Physics - established and run by the famed physicist Niels Bohr - flourished from Carlsberg Fellowships.

Niels Bohr
Physics, Beer, and Cowboys
The Institute under Niels had the unique distinction of providing support for some of the most important scientists of the 20th century. Nevertheless, Niels created an easy-going and relaxed environment. People showed up when they wanted and worked as long as they liked. When they got tired of science, they went to cowboy movies which were Niels's favorites. The Rutherford lab was also fairly easy going and in fact Ernest discouraged his students from night work as he believed evenings were more suited for tennis and croquet.
But American science changed all that and Ernest Lawrence typically put in a 70 hour work week. Soon the American spirit of - quote - "competition" - unquote - developed to where a professor - not Ernest - once cheerfully referred to his graduate students as "slave labor".
Of course, there was a reason for this shift in philosophy. The early to mid-20th century wasn't just a time of major scientific breakthroughs. There was a war on.
We mean World War I, of course. In 1914 much university activity was shelved. Otto was drafted into the army (where he worked on making poison gas), and Lise volunteered to serve as an X-ray technician. Although the war devastated Germany and launched a brief and little remembered revolution - which accomplished absolutely nothing - Lise managed OK. In 1918, she returned to the Kaiser Wilhelm Institute where she kept working with Otto. In 1925, she was appointed a professor at the University of Berlin.
Being a professor at a German university was no mean achievement. The title in Europe is not the same as in American colleges and has a prestige and importance similar to a department chairman. There were also very few women professors and very few women professors of physical sciences and mathematics. And after the war, Lise and Otto kept working on radioactivity.
Radioactivity had been discovered by Henri Bequerel in 1896, and Henri, Marie Curie, and her husband Pierre had shared the 1903 Nobel Prize in Physics for the discovery. Later and working on her own, Marie had discovered a new element that was so radioactive it actually glowed in the dark. That was radium, and that discovery landed her a second Nobel Prize and for herself alone.
By the first years of the 20th century people had figured that the radiation from radium was due to particles, and the particles were positively charged. Ernest Rutherford decided to find exactly what the particles were. So he packed a sample of radium in a tube, and put the tube in a larger tube filled with nitrogen. He figured when the positive particles zipped out into the nitrogen, they would grab an electron and change into a neutral atom. If they could identify the element, you could figure what the particles were.
Sure enough, after a time Ernest looked at the outer tube using a spectroscope and saw the tell-tale lines of helium. So the alpha particles were simply helium nuclei being shot out from the larger atoms. Today the helium we use to fill up party balloons was once alpha-particles that came from naturally occurring radioactive elements.
Of course, if radium lost a helium atom, that means it couldn't be radium anymore. Instead, it becomes the gas radon.
226Rd → 222Rn + 4He (alpha-particle)
Radon itself then decays through a series of radioactive elements until it becomes good old fashioned lead, stable and non-radioactive.
But that takes a while. What happens first is radon decays to form polonium. Then that falls apart to form a radioactive form of lead, lead-214, which decays to bismuth-214, which then forms polonium 214 - we're not loosing much mass here - and then it changes to lead-210. Some simple calculations show us that if you were to have 1 pound of radon - more than you'll ever get - in about two months you'd have 15 ounces of lead-210.
Ah, you say. Lead - nice malleable lead. Sure it's toxic, but after all, you can take care of it easy enough.
Well, there's yet another problem. The lead we're talking about is, as we said, lead-210. This isn't the lead people used in pewterware or lead pipes. Lead-210 is radioactive but it sticks around for quite a while. If you have a pound of lead-210 it takes about 300 years for it to decay down to normal non-radioactive lead. So once the radon came flying out of the radium, it formed the radioactive lead that would settle in the lab. So we see how you get the radioactive surfaces where Otto and Lise worked.
226Rd → 222Rn → 218Po → 214Pb → 214Bi → 214Po → 210Pb (Sticks Around)
Now uranium ore is not that rare and in fact had been used for thousands of years to make nice orange and yellow ceramics (you can still sometimes find these radioactive dishes around). And what made recovering radium easier is that most of the radium in the ore ends up in the "tailings" - that is waste rock and dirt that the miners just throw away. In fact, Marie Curie did her work on tailings that were given to her for free from the mining companies.
So radium soon became available to researchers and provided a nice convenient way to make alpha particles to hit other atoms. You'd pack a bit of radium into a cylinder, and bring the open end next to a piece of metal or a metal compound. Then after a certain time - sometimes minutes, sometimes hours, days, or weeks - you'd give the bombarded "target" to an analytical chemist. What, you would ask, was there that wasn't there before?
Although today a piece of routine analytical equipment can easily hit $50,000, you rarely have to isolate the element or compound to see what you've got. Of course, you have to get someone to prepare the sample and run the machine but no one denies that analysis today is easier and faster than in the good old days of Lise and Otto.
Instead what Otto and Lise had to do was to physically separate the components of a mixture from each other. Or rather their collaborator - not Ihre Mitarbeiter - the analytical chemist Fritz Strassmann had to separate the components.
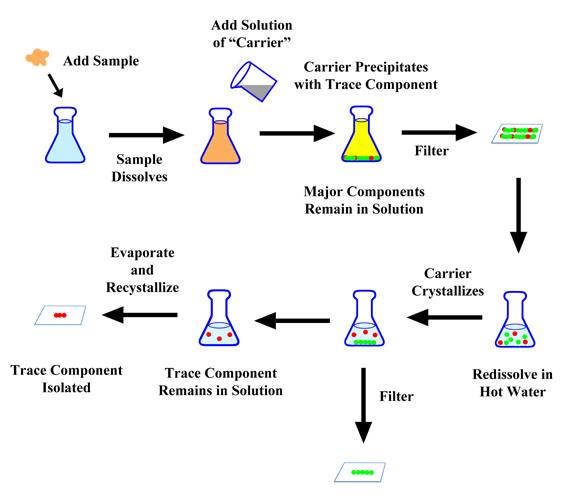
The general procedure to analyze a metal was to dissolve the compounds or mixtures in strong acids, be it sulfuric, hydrochloric, or nitric. Then you would add a second compound - usually dissolved in water or acid - which would precipitate your metal as a pure compound but leave the other stuff in solution.
Then you would take the precipitate, filter, dry, and weigh it. As long as the precipitate was a nice pure compound, you knew the amount of the metal present would be a definite percentage of the total weight. So you could then back-calculate the percentage of the metal in the original sample.
Sometimes, though, your precipitate wasn't a nice pure compound. Then you'd have to purify it. This might be simply putting the precipitate in water and heating it up until the solid dissolved, and letting the solution cool. If luck was with you, you'd get nice pretty (and pure) crystals which you could then filter. If the crystallization works as you hope, the impurities would stay dissolved in the water.
The biggest difficulty was that these analytical techniques work best for measuring metals that are present in pretty large amounts. But even naturally occurring radioactive elements are often present only in trace quantities. Radium makes up only about 0.0002 % of the uranium ore. So from ten tons of pitchblende you'll get less than half an ounce of radium metal.
The problems Otto and Lise encountered were even more formidable. If they bombarded a metal with alpha particles, they might get only a few micrograms of material - sometimes less. In principle you can do the usual analysis, but what is called gravimetric trace analysis has major challenges.
It was always notorious that trace metals in liquids often stick to and contaminate a precipitate. In normal analyses, that's not a problem since the trace components add nothing measurable to the weight. But if it's the trace elements you want and they stick to your precipitate, how do you weigh them? It would be like weighing the battleship with and without the captain.
However, it turned out the trace element contamination had an advantage. By grabbing the trace elements, the precipitate acts as a "carrier" for the radioactive material. So at least you can remove the radioactive metal from a lot of other junk that's in your original sample.
But how does that help? If the metal you want sticks to the precipitate, how can you ever isolate it? You started with a trace of your metal in a solid - and end up with a trace of the metal in your solid!
Well, there is no one procedure to handle this dilemma. One way is to redissolve the carrier with the trace compound and use crystallization (for some reason, called by chemists recrystallization). That is, boil away a lot of the water, and let the now concentrated solution cool. If you're lucky the carrier will crystallize to nice beautiful crystals. When you crystallize compounds, there is less of a tendency for the impurities to stick with the solids than on a precipitate. You then filter off the carrier leaving the trace compound in solution.
If you are after the trace elements - like Marie was in isolating radium - you would then keep collecting and combine the final trace solutions. You then evaporate the water leaving the solid and following another recrystallization, you end up with a pure compound.
That's the idea, but of course the actual procedures are more complicated. Usually you have to repeat the precipitation, redissolving, and re-precipitation sequence a number of times (five repeats is considered efficient). But with the right method you can end up with the trace metal in hand as a pure chemical compound. Then, if needed, you can convert the compounds to the pure metal. Using these exacting and difficult procedures, Otto, Lise, and Fritz had become quite famous for discovering a number of new elements and isotopes.
Lise and Otto were fortunate in that the years they worked together were when some of the most interesting scientific discoveries were being made. But there were other - ah - "interesting" developments during those years.
After World War I, Germany had gone into one of the worst financial spirals in history. Massive inflation hit the nation and from 1918 to 1923 the price of a German mark had dropped to one trillionth of the value in 1918. Most of the loss in value was in 1923 alone. A glass of beer that cost one mark in 1922 cost 5,000,000 marks in 1923. The government stamped extra zeros on the bills and even that didn't keep up with inflation. People had to spend their money as soon as they got it. If you missed a streetcar, by the time you got to the store, your money could be worth only a quarter of what it had been. Once a woman was carrying her money in a basket. She set it down for a moment and when she returned her basket was stolen and the money lying on the ground.
What caused the inflation? Well, economists say it's because the countries print too much money without increasing actual revenue. But at the time many Germans began to grumble that it was the Allies' demands for war reparations in 1918 that destroyed their economy. Still, the inflation was pretty much over by the end of 1924, and in 1925 the mark was redefined by lopping twelve zeros off the bills. So when all was said and done, the price reverted to about four to a dollar - which is what it was before the war.
But bad economics also makes for bad politics. By the late 1920's, what had been a nutty fringe group of conspiracy-theory spouting xenophobes - calling themselves the Nationalsozialistische Deutsche Arbeiterpartei (NSDAP), or in English the National Socialist German Workers' Party - had actually become a political force to be reckoned with.
In 1930, the NSDAP hoped to boost their membership in the German Parliament, the Reichstag, to over 60 seats. They won 107, and by 1932 there were 230 Nazis in the 615 seat parliament. The next year, the party leader - who had never held elected office - was appointed as German Chancellor. Then in a succession of - quote - "emergencies" - unquote - he issued proclamations that quickly dismantled the democracatically created Wiemar Constitution of 1919. One of the goals of the new leader was to eliminate Germany of all its Jewish citizens.
Lise was in a difficult situation. Although she was a practicing Lutheran she was Jewish by birth. Now before the Renaissance, if you were Jewish everyone thought of you as just a person who practiced another religion.
But in the 1400's this idea had changed in an important way. The Spanish Inquisition had decided that if you were Jewish and converted to Catholicism, that did not alter the fact that you were really Jewish. That is, people began to talk about a "Jewish race".
The racial theory grew in popularity but not everyone bought into it. King Ludwig II of Bavaria once told his friend Richard Wagner that Jews and Christians differed only in denomination. Of course, Richard didn't agree and he and his second wife, Cosima, continued to spout what was entirely racist völliger Bockmist.

Richard and Cosima
Völliger Bockmist.
Unfortunately the new leader of Germany did hold with all that völliger Bockmist. He even specified that anyone with two Jewish grandparents or anyone with one Jewish parent were Jewish. So regardless of her religion, by German law, Lise was Jewish.
But because Lise was Austrian she had been exempt from the anti-Jewish legislation. When the Nuremberg Laws of 1935 began the removal of Jewish professionals from their posts, she was able to stay at the university. But in 1938 and with the Anschluß of Austria, Lise found herself subject to German law. Realizing she was in serious danger and with the help of her friends - not just Otto but also Niels Bohr and others - she managed to escape to Sweden where she got a job as a research assistant at the Nobel Research Institute of Physics.
Although Lise later reproached herself for staying in Germany for so long, we have to remember in 1933 she was fifty-five years old. This was a tough age for anyone to pull up stakes, much less flee to another country. And like many other residents of Germany, she had hoped that somehow the country would weather the storm, and the people would come to their senses.
For his part Otto opposed the Nuremberg Laws although he never became so vocal a critic as conductor Wilhelm Furtwänger who had also decided to stay in Germany. Notwithstanding the (in)famous photograph of Hitler and Wilhelm shaking hands beneath a massive swastika, Wilhelm would argue with the dictator about the German racial policy. Once during a rather testy exchange Hitler threatened Wilhelm with internment in a concentration camp. Wilhelm snorted that then at least he would be with good company. For his part Otto tried to help his Jewish colleagues keep their jobs, albeit in the end without success.
Almost as soon as she arrived in Stockholm, Lise received a note from Otto. Otto, it seems, had run an interesting experiment. By that time everyone knew that if you took radium and mixed it with beryllium, the beryllium absorbed the alpha particles and became carbon while ejecting neutrons.
The neutron had been discovered only five years before but had already become a major tool for studying radioactivity. Since neutrons had no charge, they were easier than alpha particles to send into an atomic nucleus. A simple "neutron howitzer" was easy to make. Since wax could stop neutrons, you simply drilled out a hole in a chunk of wax - paraffin or beeswax worked fine - and put in your powdered radium and beryllium. The neutrons were stopped by the wax but shot out of the hole.
Even as late as the mid-1960's these neutron howitzers were routinely used in undergraduate lab experiments and could even be found in home labs for the interested layman. But today due to the restrictions of using radioactive compounds - not to mention their hazards - the howitzers have been much restricted in their educational use and are totally verboten for the home hobbyist.
So soon after Lise arrived in Sweden, Otto and Fritz had turned their neutron howitzer on uranium. After a suitable time of bombardment, Otto - or actually Fritz - would analyze what they made.
At the time, most of the new elements had been similar in weight to what you started off with. Maybe a few mass units higher or lower. The idea was the radiation chipped off a bit of the original nucleus as it was absorbed.
In keeping with the conventional thinking, Otto and Fritz had figured that by bombarding uranium with a neutron, they had made a new isotope of radium. That was convenient since by then everyone knew how to separate radium from uranium. It's very simple really.
First Fritz dissolved the uranium in hydrochloric acid. Then he added barium choloride. So he now had a solution of barium chloride, uranium chloride, and (he supposed) radium chloride in HCl.
Then he neutralized the acid with sodium bicarbonate. Since barium carbonate is insoluble, it also precipitated out nicely carrying with it the radioactive stuff. The uranium remained dissolved in the acid.
Then Fritz filtered the precipitate and redissolved it in hydrobromic acid (not as strong as hydrochloric) and the precipitate went into solution. He now had his mystery element - which remember he thought was radium - free of uranium.
The next step was to separate the radium from the barium. That wasn't too hard. You just boil off most of the water. The barium bromide crystallized out nicely and so the radioactive compound should remain in solution.
But what Otto and Fritz found perplexed them. The radioactive compound was not in the liquid. It remained with the barium solid. What was going on?
Well, maybe the radium was indeed being carried out of solution by the barium bromide, perhaps by adsorption. So Otto and Fritz grabbed a small sample of a soluble radium compound and added it - "spiked" is the word - to a uranium mixture. They ran through the procedure again and the radium stayed in solution. It didn't go into the barium. Their separation procedure was working fine.
So whatever the uranium produced stuck with the barium through thick and thin. It was, in fact, acting like radioactive barium.
But barium was way, way, way lower in atomic weight compared to uranium. Making barium went against the accepted wisdom of what happened in nuclear reactions. Wondering what was going on, Otto had written to Lise if she had any ideas.
Lise, too, was perplexed. Like everyone else she thought in terms of the neutron chipping away at the nucleus. That should make radium or at most something no lighter than thorium.
Lise's nephew, Otto Frisch, was in Sweden, too. He was working with Niels Bohr in Copenhagen and would soon move to England. But now, knowing his aunt was alone, he decided to spend Christmas with her.
When she told Young Otto about the experiments he couldn't believe it. Hans and Fritz must have made a mistake, he said. Lise, though, thought that Otto and Fritz knew what they were doing. It looked like they were making barium.
Otto decided to change the subject and said he'd like to try out his new cross-country snow skis. Lise said she'd walk along beside him (Lise was a pretty fast walker).
After a while they took a break and sat down on a log. Lise brought up the uranium experiments. After a while, Lise asked about the liquid drop model of the nucleus which had been proposed by the Russian physicist George Gamow and championed by Niels Bohr.
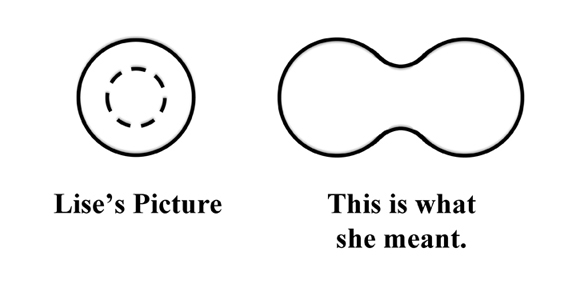
The model treats the nucleus as a liquid drop. Normally a drop is spherical. But if it gets too large, it stretches out. A big enough drop will eventually pull itself apart and break roughly in half. Lise sketched a circle with a dotted circle inside and asked if this could be what was happening. Otto wasn't sure what his aunt was trying to draw and he sketched out a dumb bell shape. Lise said, yes, that was what she meant. If hit by a neutron, could the nucleus distort to a dumbbell and split in half?
Although every grade school kid knows that like charges repel, the truth is this is a simplification. For instance, if you take two cylindrical magnets, and try to push identical poles together, they repel each other. On the other hand, if you put the magnets in a tube and smack the top one with a hammer, they will mush together.
Of course, what happens is the force of the blow is strong enough to overcome the force of magnetic repulsion. And when the magnets smash together the interatomic attractive forces overcome the magnetic repulsion and the two pieces of metals stick together.
That's sort of what happens with mashing atomic nuclei - made up of neutrons and protons. The protons will repel each other if they are about 0.00000000000004 inch or further from each other. Smash them together to 0.000000000000039 of an inch and they actually attract. Neutrons have no charge but if they get close enough they will also attract other neutrons and protons. If you smash protons and neutrons close enough together, hey, presto!, you have an atomic nucleus.
Now lest particle physicists go into spittle-flinging diatribes, we know what we said and what follows is a gross oversimplification. But as Albert Einstein might have said, pech gehabt!
We see that if you have a nucleus small enough, the protons and neutrons are close enough so the attractive forces overcome the positive repulsion of the protons. But if you make a nucleus too large - too many protons and neutrons - then the positive charges of the more distant protons would repel each other, and you wouldn't have a stable nucleus.
So there must be some element that is just small enough that the protons attract each other, but if the nucleus absorbs just one more proton or neutron, it would be just too big to be stable.
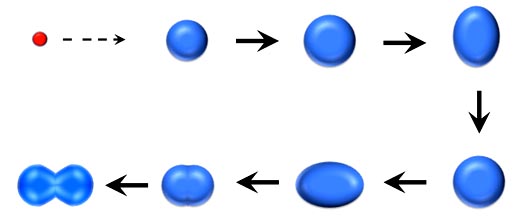
At that point the liquid drop model - which even today is not a bad way to look at a nucleus - can come into play. If you were to hit a liquid drop with a smaller drop, it doesn't keep its nice spherical shape. It distorts and and starts to bulge, deform, and oscillate. And one of the deformations will be - yes - like a dumbbell.
But now look at the dumbbell shape. You have the two balls - "lobes" is a better word - held together by a narrower tube. But if what Lise said was actually happening - the nucleus would begin to act like a drop and distort to the dumbbell shape. Then the protons in the two lobes would be too far apart to attract each other and the electrostatic repulsion between positive charges would take over. The lobes would then go flying apart, and the nucleus would split into to nearly equal halves.
Suddenly everything made sense. The reason you didn't have any naturally occurring atoms larger than uranium was because its nucleus was just small enough to be stable. So add that one more neutron and some protons would be too far apart for stability. The nucleus begins to distort and at some point forms the dumbell shape. The atom then splits.
Now if the nucleus was actually tweft in twain - that is half of the 92 protons going one way and the other half the other, you'd get two atoms with 46 protons. This would mean you would get two palladium molecules. But half of uranium's atomic weight - which is 238 - is 119. But there is no palladium 119.
Of course, theory is fine, but experiment is the real world. Since Otto and Fritz were getting a radioactive isotope of barium, then that's the way it must be. Barium's atomic number is 56 and since 92 - 56 = 36, the other atom should have atomic number 36. That's krypton.
So if uranium split into barium, you should also get krypton. Krypton, though, was a gas. It wouldn't be picked up in the analysis. It would float away.
So that much was clear. Otto and Fritz should also look for krypton. But the total mass of krypton and barium was 221 units which is quite a bit less than the weight of uranium. So Lise and Otto knew that the nucleus must also be breaking down further, probably into more neutrons.
Lise, Young Otto, Otto, and Fritz corresponded back and forth. Otto and Fritz said they would write up and describe their experiments and Lise and Young Otto would also write a separate article explaining what's going on.
But why, couldn't they just write one article? That would save time and everything would be tied into one nice neat package.
Some people have suggested that Otto wanted to hog all the credit himself. But that's not, to paraphrase Arlo Guthrie, very likely, and we don't expect it.
Remember, Otto was working in Nazi Germany with the Nuremberg Laws in full force. Whether he wanted to have Lise as a co-author couldn't overcome the sad fact that no paper written by a German academic would be permitted to have a Jewish co-author. This was the time that Germany would not even print a German edition of The Hobbit unless they received written confirmation that the author, J. R. R. Tolkien, was not Jewish. For his part, Ronald told his own publisher that he'd just as soon let a German edition go and hang. But he did write a rather stiff note saying that regarding Jewish ancestry he regretted that it appeared that he had no ancestors of that gifted people. He closed by reminding the German publisher that applying German laws to citizens of other countries was improper.
So Otto, Fritz, Lise, and Young Otto published in separate but coordinated papers. The one by Otto and Fritz was in Naturwissenschaften and was about the experiments where they expressed bewilderment on what was happening. The other paper - published in the English journal Nature - was by Lise and Young Otto where they explained what fission actually was.
By taking the two-article approach, they actually better defined credit for everyone concerned. Otto and Fritz established experimentally that the atom had been split, and Lise and Young Otto established their priority in explaining the theory of nuclear fission.

J. Robert Oppenheimer
He acted like a fuddy-duddy.
You sometimes read about how old fuddy-duddy establishment scientists resist new theories. Actually, that's rarely the case. Good data and good explanations almost always carry the day with good scientists (politicians are a different matter). Particularly when other people can independently and reliably reproduce the experiments and the calculations.
Luis Alvarez, a physicist at Berkeley, read the news of the atom being split while getting his hair cut. He immediately - in "mid-snip" he said - left the barbershop and and told the department chairman, Robert Oppenheimer, about the work. Here Robert did act like an old fuddy-duddy. He went to the blackboard and quickly - quote - "proved" - unquote - atomic fission was impossible.
Luis, though, went to his lab and repeated the experiment. He didn't analyze the mixture but set up a detector to capture subatomic particles. The oscilloscope showed blips that could only be the products of the split nucleus. Luis went and called Robert down to his lab. Robert not only realized that the atom had been split but with enough uranium, the neutrons produced would hit more uranium atoms. That could start a chain reaction, and the splitting would take off on its own. So atomic fission could, Robert said, provide power or ..... make a bomb.
When the news hit the rest of the scientific community, there was the inevitable "Why didn't I think of that" response. Glenn Seaborg, the Berkeley chemist who, like Otto and Lise, had gained fame for creating new elements, kicked himself for missing what had been going on. Niels Bohr, who had helped develop the liquid-drop model of the nucleus, slapped his forehead and cried, "Oh, what idiots we have all been!"

Glenn Seaborg
He kicked himself.
Now one thing Lise and Young Otto also did was to calculate how much energy would be released. After all, you have two oppositely charged particles produced and they go flying apart. This releases energy.
How much energy?
Lise and Young Otto's paper is what's called in the scientific literature a "note" or "communication". In notes or communications, you don't usually give many details. But they said that the amount of energy released would be about 200 megaelectron volts - or 200 MeV. So naturally we would like to see how Lise and Young Otto got that number.
I thought you would as Captain Mephisto said to Sidney Brand. It's very simple really.
If you look on the Fount of All Knowledge to find where the energy from atomic fission comes from, you'll get different answers. The glibbest answer is it's from Einstein's equation E = mc2. Since the speed of light is so large, you get a lot of energy from a little mass.
But others - including Nobel Prize Winner Robert Serber - will tell you that atomic fission is a non-relativistic phenomenon. That is, you don't need to use Einstein's equation to calculate the energy. Classical theories work fine, thank you. But as we'll see, both explanations are correct.
The point is that there is no such thing as a non-relativistic phenomenon. Everyone is subject to the principles of relativity. There are only non-relativistic calculations. But if the non-relativistic calculations pretty much give you the right answer, you can say you're looking at a non-relativistic phenomenon.
The truth is any loss or gain of energy causes a change in mass just like Albert's equation says. For instance, if you get in your car and burn up 10 gallons of gas, you'll use up about 30,000 kilocalories - the "calorie" of food scientists. If you take Albert's equation:
E = mc2
... and rearrange it, we get:
m = E/c2
So using the speed of light in meters per second, 299792458, and after converting the kilocalories to joules - the standard scientific units of energy - we end up with the kilograms lost by the energy:
| Mass | = | 125520/2997924582 | |||
| = | 125520/9000000000000000 | ||||
| = | 0.000000000014 kilograms | ||||
What this means is that if you were to weigh all of the products of combustion - the carbon dioxide, the water, and all of the other sundry products - they would weigh 0.0000000005 ounces less that the gasoline you started out with.
Of course, you can't weigh the products that carefully. But in atomic fission much more energy is given off and so we'll see you can measure the actual mass lost as the fission products are pushed apart by the positive repulsion. So we can exercise both options if we just:
- Determine how much energy is released when the two nuclei are forced apart by the positive repulsion of the newly formed nuclei.
- Calculate the difference in mass of the uranium and the fission products and then calculate the energy equivalent of the lost mass using Albert's equation E=mc2.
First we'll use the Coulombic energy approach. That's what Lise and Young Otto did.
Now everyone knows that the force between two charged particles is given by Coulomb's Law.
Force = kq1q2/r2
We won't go through the simple calculus involved, but the potential energy of these two charges is simply:
Energy = kq1q2/r
So all you need to know is the charge of the protons, the value of the constant (which gives us the right units), and the distance separating the two charges.
Yes, that's all you need to know.
But even in the early 20th century, we knew the values good enough. The mass and charge of the electron were both known by 1910. And the charge of the proton was found to be equal to that of the electron since people knew hydrogen had one proton and one electron.
Now we need the distance between the charges
It was known since about 1920 that the nucleus was small compared to the diameter of the atom. This was shown by Ernest Rutherford when he sent a stream of alpha particles - generated by radium - through a thin gold foil. Most alpha particles went right through the foil but others were deflected. By measuring the number of alpha particles that got bounced at what angles, he determined that gold's nucleus had radius less than 0.000000000000027 meters. That's 27 × 10-15 meters or 27 femtometers or more briefly, 27 fermis.
This, though, was an upper limit. Since the nucleus was positive and the alpha particles were postive, they repelled each other. So you could only get them so close. Ernest figured if he used a lighter element - like aluminum - the lower positive charge of the nucleus would mean you would would get closer and more accurately measure the radius.
And sure enough you did. Using aluminum Ernest found the alpha particle came within about 5 femtometers of the atom's center.
The liquid drop model also suggested the nucleus should be spherical and that the volume of the nucleus wouldn't be too much different than the total volume of the individual protons and neutrons. Since the sum of the protons and neutrons is just the atomic weight and knowing a sphere's volume is proportional to the cube of the radius, our equation is:
Radius = r0A1/3
... where R is the radius of the nucleus, A is the atomic weight (A1/3 is the cube root, of course), and r0 is a constant that has to be determined.
And how do we determine r0?
Actually that's easy. Just rearrange the equation so that:
Radius = r0A1/3
... becomes ...
r0 = Radius/A1/3
And we just plug in Ernest's value for aluminum which has an atomic weight of 27, and hey, presto, we get:
r0 = 5/271/3 = 5/3 = 1.67 femtometers
So our equation for a nuclear radius is simply:
Radius (femtometers) = 1.67 × A1/3
... where you keep in mind that
1 femtometer = 1 × 10-15 meter
Next we know that krypton and barium were produced. Now neither Lise nor Otto nor Fritz nor Young Otto knew exactly what isotopes they had. So you can just pick the most common ones which would be barium-137 and krypton-84. Plugging in the numbers we get the radii as:
| Radius (barium) | = | 1.67 × 10-15 × 1371/3 | |||
| = | 1.67 × 5.2 | ||||
| = | 8.4 femtometers | ||||
| Radius (krypton) | = | 1.67 × 841/3 | |||
| = | 1.67 × 4.4 | ||||
| = | 7.1 femtometers | ||||
So once the uranium splits, the centers of the two nuclei will be separated by:
| Distance (Barium-Krypton) | = | 8.4 + 7.1 | |||
| = | 15.5 femtometers |
... or in standard form ...
Distance (Barium-Krypton) = 1.55 × 10-14 meters.
OK. So how much energy will be released? Remember it's the same as the potential energy of the two atoms before they start to fly apart. So we go to the formula:
Energy = kq1q2/r
... and with 56 protons in barium and 36 protons in krypton and the values for the other constants (which you can look up easy enough).
Energy (in joules) = (9 × 109 × 56 × 1.6 × 10-19 × 36 × 1.6 × 10-19)/1.55 × 10-14
... which is ...
Energy = 3.0 × 10-11 joules
Since there 6.2 × 1018 electron volts (eV) per joule, we have:
| Energy | = | 3.0 × 10-11 joules × 6.2 × 1018 joules per eV | |||
| = | 186,000,000 eV |
... which if you convert to mega electron volts (MeV) we get:
| Energy | = | 186,000,000/1,000,000 | |||
| = | 186 MeV |
... and since Lise said about 200 MeV, what we calculated is the right number.
Now we used the numbers that Lise would have used given the state of knowledge at the time. But if you're interested, the most up-to-date value for the nuclear radius is more like
Radius (femtometers) = 1.25 × 10-15 × A1/3
So if you want, you can prove that the updated number for the energy is more like 244 MeV. Which again is - as Lise said - about 200 Mev.
Now we mentioned you can also calculate the energy using E=mc2. That's actually pretty easy but a bit of background is in order.
Now, if you ask a chemistry student what is the atomic mass of an element with 92 protons and 146 neutrons, they'll just add the numbers up and tell you 238. That is, it would be uranium.
However, saying you can just add up the protons and neutrons and get the atomic weight is not quite true. The atomic weight (yes, yes, we know we really should say atomic mass) is not the sum of the protons and neutrons. It's not even the sum of the weights of the protons, neutrons, and electrons. And this was found out pretty early when they started weighing atoms.
Yes, weighing atoms. In 1919, Francis William Aston - who had been a graduate student at Cambridge with J. J. Thompson (the discoverer of the electron) - was looking at how atoms changed direction when you knocked off an electron and sent them through a magnet. Because the atom has become an ion - that is, it has a charge - the magnetic field exerts a force on the ion. And as we all know a force on something will cause it to accelerate.
But not necessarily change speed. A change in direction is also an acceleration. So if you sent the ion through the magnetic field it will curve. And the math of electromagnetism tells us the amount of the curve depends on the atom's weight. So if you keep the magnetic field constant and zap the ions into the magnet, the different ions of different weight curve by different amounts.
By the mid-1930's Francis and other physicists - including Kenneth Bainbridge who later was the director of the test of the first atomic bomb - had instruments that could measure atomic masses to 0.00001 of an atomic mass unit. In other words, although we know the atomic weight of hydrogen is about 1, if you round the number off, when measured to five decimal places, it comes out to 1.00797.

Kenneth Bainbridge
He weighed atoms.
These new high powered mass spectrometers could also measure the mass of the protons. Since they were charged particles they also curved in the magnetic field. The mass of the neutrons, though, had to be calculated indirectly and in fact the neutron wasn't even discovered until 1932. But once people realized that atoms were made up of protons, neutrons, and electrons, they could in principle add them all up and compare the calculated weight with the experimental weight.
Well, we'll get a bit ahead of ourselves. But Lise, Otto, Fritz, and Young Otto didn't realize that the uranium that was splitting wasn't your everyday uranium which has an atomic weight of 238. Nor did it split into ordinary barium with atomic weight 138 or normal krypton with mass 84. Instead it was uranium-235 - a less stable isotope - splitting to barium-141 and krypton-92 with two neutrons balancing out the differences.
So let's tally up the weights of everything and see how they balance out.
The uranium-235 nucleus weighs 235.043925 atomic mass units (also called amu). Barium-141 weighs 140.9144119 amu and krypton-92 91.9261561 amu. We toss in one neutron to start the splitting and get three back for a net loss of 2 neutrons. One neutron weighs 1.0086649158849 amu.
So the net weight difference is:
| Difference | = | 235.0439299 - 140.9144119 - 91.9261561 - 2 × 1.0086649158849 | |||
| = | 0.186032068 amu |
In other words, the uranium-235 weighs more that the sum of the fission products. Or alternatively, if the atom splits into barium-141 and krypton-92, you lose some of the uranium's mass.
Now the mass lost is not huge but it is certainly not simply experimental error. And that extra weight has to go somewhere, And it does so by becoming energy.
So how much is this in energy?
Well, 1 amu is about 1.66053904020 × 10-27 kilograms.
So with c = 299792458 meters per second (exactly), we get the energy as:
| E | = | mc2 | |||
| = | 0.186032068 amu × 1.66053904020 × 10-27 kg/amu × 299792458 meters per second | ||||
| = | 2.7763762 × 10-11 joules |
... and we all know:
| If Energy | = | 2.7763762 × 10-11 joules | |||
| Then Energy | = | 173.288 meV |
So given the approximation of our Coulombic calculations 173 and 183 are close enough. And so we can round both up to the 200 meV as in Lise's paper.
OK, we've got 200 MeV from splitting a uranium atom. Just how much energy is this?
Despite the use of mega in the word, it's not much. A mega electron volt is 0.00000000000000004 food-calories (ergo, a "kilocalories" to scientists and engineers). So splitting an atom of uranium would raise a pan of water maybe 0.00000000000000001 degrees Fahrenheit.
Huh! A trivial amount of energy, nicht wahr?
Weeeeeeeellllllll, not quite.
Remember we're talking about one atom. And in one ounce of uranium you have 70,000,000,000,000,000,000,000 atoms. So an ounce of uranium will raise a pan of water 1,000,000,000 degrees Fahrenheit.
Of course the water would boil away long before that. So a better way to look at it is that a pound of uranium atoms splitting apart would give off as much energy as an explosion of 9000 tons - that's nine thousand tons or nine kilotons - of dynamite.
That's a big bang.
There was, though, a problem. The bombardment of the uranium with the neutrons was a slow process. If you took your neutron howitzer and turned it on a bit of uranium, then after a few days you might have a few thousand atoms split. To get the ounce of uranium to split would take a long time.
But remember that Robert Oppenheimer also pointed out to Luis Alvarez that it took only one neutron to split the atom and produced krypton and barium. But this fission also produced a number of extra neutrons for the weights to balance out. But these new neutrons would bump into more uranium atoms and so more atoms would split and release even more neutrons. So if you started off with only a single atom being split and which produced only 2 neutrons, in about 85 steps you could generate enough neutrons to split nearly fifteen pounds of uranium.
That, though, assumes all neutrons that get generated go on to split another atom. This isn't the case. Only a certain percentage get "captured" and split an atom. Also if you have too little uranium the neutrons will escape out of the sample before they get captured. So you need to figure out how much uranium you need to start a self-sustaining nuclear reaction.
And just how much was this?
Rudolph Peierls, a German physicist who was living in Britain, decided to look into the question. He had developed an equation for calculating how much of an element you needed to get it to start a self-sustaining nuclear reaction. So Rudolph plugged in the numbers for uranium and found that it was a lot - a minimum of 10 tons and more likely nearer 40 tons. So it didn't look like it was practical to use uranium to provide power, much less build a bomb. Figuring others would be interested, Rudolph published his findings.
The problem was Rudolph, like everyone else, figured it was common everyday uranium that was absorbing the neutron. But then Young Otto - now in England after the Nazis invaded Denmark - was working with Rudolph. From Niels Bohr, Otto had learned it was more likely it was U-235 that was splitting, which we know it was.
In fact, the atomic properties of U-235 suggested it was about 10 times more likely to split than U-238. That still didn't seem like enough to make any practical difference in the amount needed to make a bomb. But when Otto and Rudolph put the numbers for U-235 into Rudolph's equation, the amount needed - instead of being tons - turned out to be about a pound - the size of a golf ball.
But there was another difficulty. If the U-235 was at its normal concentration - about 0.7% in the U-238 - that was too low for the reaction to kick off and run on its own. You had to "enrich" the uranium. For a bomb, you needed around 85 % pure U-235.
But remember, we know that you can't separate isotopes by chemical refining. They stick together as one element.
But you could use physical methods to separate - or at least enrich - one isotope over another. For instance, you could make a uranium compound that was a gas and let it diffuse through a membrane. The higher atomic weight U-238 compound moves slower than the lighter U-235. Not much slower, true, but if you had a series of chambers separated by membranes, then the purity of the U-235 would increase in each chamber. With enough membranes, you could get as pure U-235 as you wanted.
Again Young Otto sat down and did some calculations. It would take a lot of membrane tubes, yes - but they could still be installed in a large but still practical industrial plant. You could make a few pounds of essentially pure U-235 in a few weeks.
Well, we know the story. The physicist Leo Szilard wrote a letter to Franklin Roosevelt - then the US president - and asked Albert Einstein to sign it. When Albert had been told about the possibility of making fission bombs, he had said, "I hadn't thought of that!". But he signed the letter anyway.
Rather than mailing the letter, Leo handed it to his friend Alexander Sachs, who was a friend of Franklin. Alex met with Franklin who agreed they needed to start a project looking into making an atomic bomb.
Well, the atom bomb was made and used, not once but twice. The first on August 6, 1945, the last three days later on August 9. The next day Japan announced it would surrender.
Hans had stayed in Germany. Like many of the German scientists and engineers he was arrested by the Allies and imprisoned. Hans was sent to England.
Now "imprisoned" carries connotations that don't really fit Hans's situation. "Interned" is a better word. He was kept in a dormitory with other scientists. While there he learned he had been awarded the Nobel Prize "... for his discovery of the fission of heavy nuclei."
Now it seems that the committee was slighting not only Fritz, but Lise and Young Otto as well. Fritz's contributions as an analytical chemist was crucial to determining that it was barium, not radium, that was being made. And Young Otto's looking into the consequences of the drop-model was likewise a major factor in he and Lise figuring out what was going on.
But who, in fact, had actually "discovered" nuclear fission? Although it was clearly a collaborative effort, Otto was what we call the principle investigator. The primary work was in his laboratory and under his direction. Lise and Young Otto, on the other hand, explained what went on. So shouldn't they still be considered at least co-discoverers?
The main problem was that to award all four the Nobel was not possible. There cannot be more than three people sharing a given prize. If there's too many people, you usually give the award to the principle investigator.
Today articles have asterisks by the names of the principle investigators. But that wasn't common before World War II. On the other hand both Otto and Lise were listed on their publications first - indicating they were the principle authors. So it would have been most reasonable to give a shared prize to Otto and Lise
Lise, though, said that Otto Hahn definitely deserved a Nobel Prize. But as far as sharing it, she would not have wanted to do so unless her nephew Young Otto was also recognized. And then there's people who said that awarding the Prize only to the elder Otto would have short changed Fritz who had developed and carried out the chemical separations.
The alternative - and in fact the best choice - would have been to award the Chemistry Prize to Otto and Fritz and the Physics Prize to Lise and Young Otto. The advantage of this approach was that you didn't have to give all four awards the same year - I. I. Rabi got the Physics Prize in 1944 - and instead Otto and Fritz could get the Prize one year and Lise and Fritz another. This, though, wasn't done and Lise and Young Otto never got a Nobel.
Lise settled in England at age 66, and after the war her health began to decline. Like many people who were watching a younger generation she was perplexed by some things they said. She kept a notebook, not only for writing down the new terms of physics - like "spin-spin relaxation effects" and "nuclear form factor", but also for noting incomprehensibilities like "highfalutin'" and "juke box".
Lisa lived until 1968. In 1966 along with Otto and Young Otto, she received the Fermi Award but was too ill to make the trip. So Glenn Seaborg took the time to go to Cambridge and to make the presentation.
References
Lise Meitner: A Life in Physics, Ruth Lewin Sime, University of California Press (1996).
"Lise Meitner", Atomic Archive, http://www.atomicarchive.com/index.shtml.
"Lise Meitner", Jewish Woman's Archive, https://jwa.org/encyclopedia/article/meitner-lise
The Making of the Atomic Bomb, Richard Rhodes, Simon and Schuster, (1986) The most comprehensive history of the history of the Atomic Bomb.
The Los Alamos Primer: The First Lectures on How To Build an Atomic Bomb, Robert Serber, University of California Press, 1992. Robert specifically calls nuclear fission a "non-relativistic" phenomenon
Thirty Years That Shook Physics - The Story of the Quantum Theory, George Gamow, Published by Anchor Books (1966) Anchor Books, 1966
An Introduction to Nuclear Reactor Theory, C. E. Iliffe, University of Manchester Press, 1984.
American Prometheus: The Triumph and tragedy of J. Robert Oppenheimer, Kai Bird and Martin J. Sherwin, Knopf (2006). A good book about Oppie with a lot about the development of the bomb.
The Chemistry of Uranium: The Element, Its Binary and Related Compounds, Joseph Katz and Eugene Rabinowitch, 1951.
Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology, Jean-Louis Basdevant, James Rich, and Michael Spiro, Springer, 2005.
Radium Removal from Uranium Ores and Mill Tailings, S. R. Borrowman and P. T. Brooks, U.S. Bureau of Mines Report of Investigations no. 8099, 1975.
Isolation of Uranium Mill Tailings and Their Component Radionuclides From the Biosphere: Some Earth Science Perspectives, Edward Landa, Geological Survey Circular 814, US Geological Survey, 1980
Modern Physics, Raymond Serway, Clement Moses, and Curt Moyer, Harcourt, 1997.
The Conceptual Completion and Extensions of Quantum Mechanics 1932-1941, Springer, Jagdish Mehra
Physics for Science and Engineers, Raymond Serway and John Jewett, Brooks/Cole, 2014.
Twentieth Century Physics , Laurie Brown, 3 Volumes, Institute of Physics Publishing, 1994
"Pioneering Nuclear Science: The Discovery of Nuclear Fission", Michael Madsen, International Atomic Energy Agency, December 20, 2013.
"The Discovery of Fission (1938)", LibreTexts, Chemistry, http://chem.libretexts.org/Exemplars_and_Case_Studies/ChemCases/Nuclear_Chemistry_and_the_Community/04._The_Discovery_of_Fission_(1938).
"The History of Nuclidic Masses and of their Evaluation", Georges Audi, International Journal of Mass Spectrometry in the Honor of the 65th Anniversary of Jurgen Kluge's Birthday, Issue 251, Elsevier, pp. 85-94, 2006.
The History and Science of the Manhattan Project, Bruce Reed, Springer, 2013.
The Letters of J. R. R. Tolkien, J. R. R. Tolkien, Houghton Mifflin, 1981. This has one of the two letters that Ronald wrote to the German publisher of The Hobbit. It is very clear Ronald had no truck with anti-Semitism.
The Discovery of Subatomic Particles, Steven Weinberg, Scientific American Books, W. H. Freeman and Company, 1990.
History of Oxford University: Nineteenth Century Oxford, Part 1, M.G. Brock and M.C. Curthoys (Editors), Clarendon Press.
"Three Myths about Scientific Peer Review", Michael Nielsen. January 8, 2009. Web.
The Double Helix: A Personal Account of the Discovery of the Structure of DNA, James Watson, Atheneum, 1968. You get a hint of 1) the relaxed environment at the 1950's university and 2) the relative ease of getting published prior to 1960. That said, in the Official CooperToons Opinion a refereeing system is needed more than ever given the whacko pseudoscience you get today and that the Official CooperToons Experience referees do not hinder publication as long as you have a decent paper. The main area for improvement is that both the referees and editors need to remember that the instruction that referees should "return the article with comments in two weeks" does not imply a margin of error of six months.
Return to Lise Meitner Caricature
Return to Otto Hahn Caricature